About EnCI
The Energy Conversion Interfaces (EnCI) group investigates the mechanisms, surfaces, interfaces, and fundamental processes controlling electrochemical and catalytic energy systems.
We combine advanced in-situ and operando spectroscopy, electrochemical methods, computational modeling, and modern data infrastructure to understand and accelerate the development of sustainable energy technologies.
This website serves as a central entry point to the group’s infrastructure, including:
- A database for storing experimentally optained data.
- Electronic Lab Notebook for keeping track of experiments.
- Tools for collaborating on projects and sharing data.
- Instrument documentation, protocols and guides.
Research Focus
Finding a scalable acid-stable water oxidation catalyst
Hydrogen is certain to play a role on a terawatt scale (720 GW by 2030 and 3.7 TW by 2050 according to the IEA), and the industrial bottleneck for green hydrogen is the ability to install energy-efficient water electrolyzers quickly and at scale. In the best available technology for producing green hydrogen from intermittent renewable electricity, polymer electrolyte membrane (PEM) electrolyzers, most of the energy lost today is due to water oxidation on the anode by the oxygen evolution reaction (OER), 2 H₂O → O₂ + 4 (H⁺ + e⁻), and this reaction at present requires the extremely rare material iridium oxide (IrO₂) as electrocatalyst.
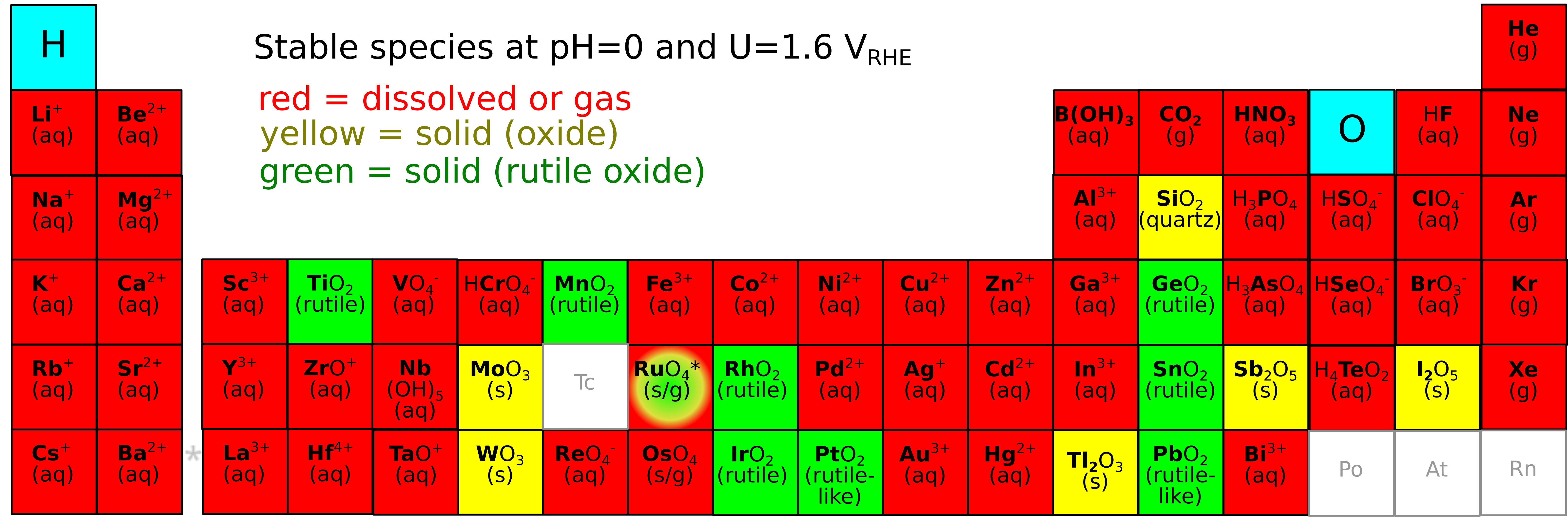
Replacing iridium is challenging due to the instability of most elements in the acidic and oxidative conditions at the PEM anode. Of the stable and abundant elements, none are good catalysts in their pure form. However, these elements form a multi-dimensional composition space of high-entropy oxides (mixes of four or more metals plus oxygen) which is likely to contain active, stable, and scalable PEM electrolyzer catalysts. Our group will search this composition space using electrodeposition synthesis, EC-MS testing, in-situ spectroscopy, and machine learning algorithms, with an emphasis on determining how the electrocatalytic mechanism varies with composition.
Novel in-situ methods
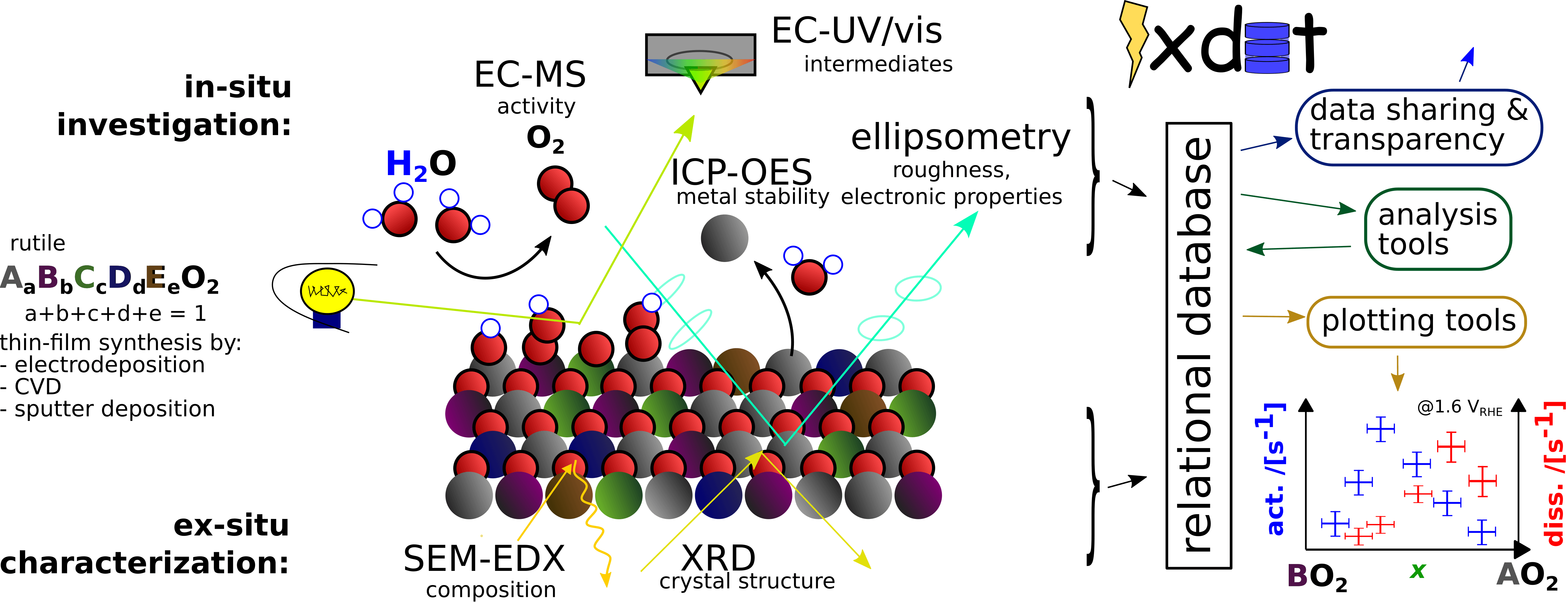
Discovery and optimization of the best electrocatalysts for water oxidation, or any other reaction, is facilitated by knowing how the reaction proceeds at an atomic level. Likewise, improving the stability of electrocatalysts is facilitated by knowing how degradation proceeds at an atomic level. Detecting the reaction products real-time with high sensitivity is one way to do this. With electrochemistry–mass spectrometry (EC-MS) we accurately measure the rate of any gas-producing electrochemical reaction including water splitting to O₂ and H₂ and carbon dioxide reduction to C₂H₄, for example. We can also probe electrocatalyst surface reactivity for other reactions including biomass valorization with stripping experiments, which release sub-monolayer amounts of CO₂.
While quantification of the reaction products can be used to infer insight in the electrocatalytic intermediates, actually observing them as a function of potential will bring more insight to the mechanism. For this, we will build and use in-situ UV-Vis (spectroelectrochemistry, SEC) to observe and quantify electrocatalytic intermediates.
Open-source python package
The data generated from the in-situ methods described above is often complex, coming from different instruments. Often, the
datasets need to be lined up in time before they can be analyzed together, which can be tedious. Furthermore, the presentation
of experimental data from these techniques is not standardized, and the data is rarely shared in an easily usable way as
necessary for open science. We have developed the in-situ experimental data tool (ixdat), an open-source python
package, to help counter these problems. We plan to expand this as a platform for in-situ techniques in general, and as a
database system to connect experimental and computational data on electrocatalysis in a shared easy-to-use system.
Contact & Location
EnCI Group
Department of Chemistry
Technical University of Denmark (DTU)
For inquiries regarding collaboration, infrastructure, or documentation, please contact the group administrator or PI through internal channels.